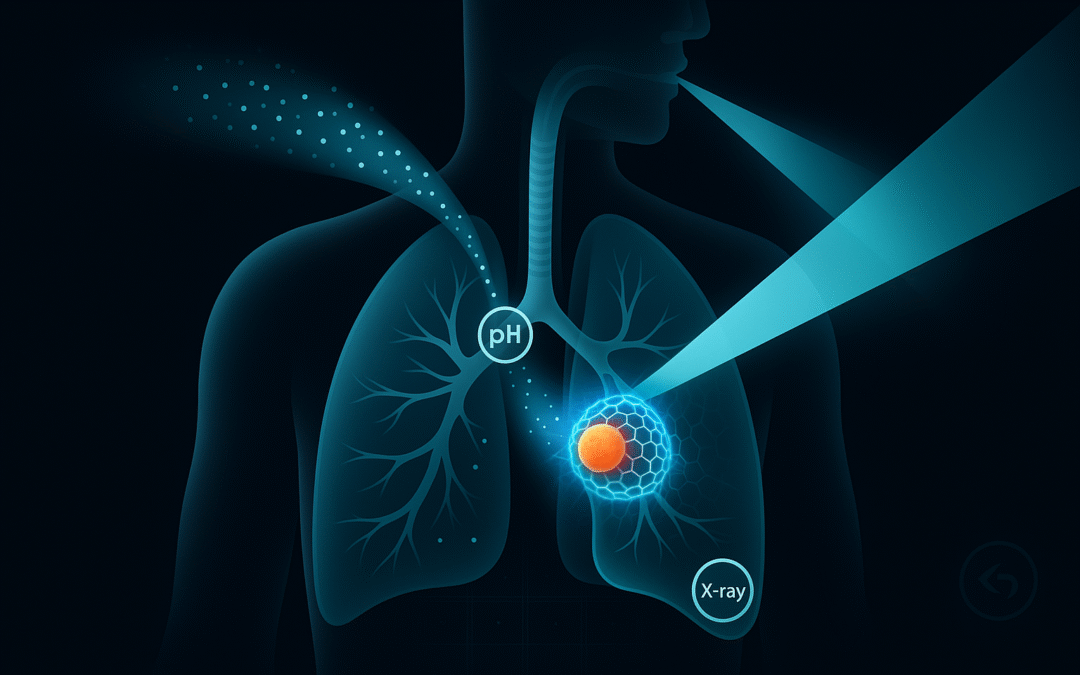TL;DR
We propose an inhalable nanoparticle system that biases toward lung-tumor regions (via pH-sensitive retention) and, when exposed to clinical X-rays, polymerizes in situ into a thin hydrogel “mesh.” The intended role is adjunctive: help limit cell shedding at tumor margins during/after radiotherapy, optionally carry small drug or radiosensitizer doses, provide on-table imaging cues, and include a reversible “undo” mechanism. This is a concept for discussion and evaluation—not a clinical product.
How this started (August 2024)
The original note outlined a simple, two-gate architecture:
-
Gate A — Targeting. Aerosolized nanoparticles tuned for deep-lung deposition with a pH-responsive shell to increase residence near acidic tumor microenvironments.
-
Gate B — Assembly. Particles co-carry latent monomers/crosslinkers and an X-ray–harvesting trigger (nanoscintillator or radiosensitizer). Under standard radiotherapy beams, local initiation would crosslink a thin peritumoral hydrogel.
-
Intended function. Provide a physical containment layer at the interface between tumor and airway; optional local payloads.
-
Safety idea. Require both gates (tumor pH and X-ray) and keep an emergency reversal path.
That was the core: inhale → bias → assemble → contain → reverse if needed.
What we’ve updated (and why)
Since that first draft, several independent advances have appeared that inform the proposal:
-
Studies have shown X-ray–initiated hydrogel formation in vivo using scintillating or persistent-luminescent components (proof that deep-tissue initiation is possible).
-
Separate work has demonstrated inhaled airway-tolerant hydrogels in large animals, suggesting that benign coating of airways is achievable under controlled conditions.
-
Inhalable “trigger-logic” platforms (different application) have validated the aerosol-in → local chemistry → external workflow pipeline.
-
Additional publications describe reversible or degradable crosslinks addressable by light/ROS/redox, which could serve as a de-gelation mechanism.
-
Imaging-visible hydrogels provide practical ideas for on-table verification (e.g., CT contrast, optical afterglow).
None of these is our integrated system; together they help frame a more realistic specification and testing plan.
The proposal now (v2.0)
Objective. Evaluate whether an inhalable, X-ray–triggered hydrogel can form a thin, conformal layer around lung-tumor margins to support local control during radiotherapy.
Gate A — Targeting & deposition
-
Aerosol MMAD ~1–3 µm for distal airways.
-
pH-responsive outer layer (e.g., imidazole/β-amino ester motifs) for biased retention; PEG/zwitterionic brushes for mucus navigation.
Gate B — Programmable assembly
-
Trigger: nanoscintillator (X-ray → visible) or radiosensitizer (short-range radicals).
-
Chemistry: lung-benign PEGDA/thiol-ene mixes, held below gel point until local trigger intensity exceeds threshold under routine planning doses.
Function options
-
Containment: peritumoral “shrink-wrap” to help limit detachment/transport of cells.
-
Adjuncts (optional): micro-dose drug, radiosensitizer, or immuno-adjuvant embedded in the gel.
-
Imaging: low-level CT dopant and/or persistent luminescence for simple intra-procedure checks.
Safety & reversibility
-
Dual-gate logic (pH and X-ray), conservative monomer levels, lung-compatible backbones.
-
Reversal: cleavable crosslinks (photodegradable or redox/ROS-labile) as a contingency if airway patency is affected.
Evaluation path (high level)
-
Bench/ex-vivo studies for gel thresholds, rheology, airway epithelium compatibility, mucociliary metrics, and dose-to-gel maps under clinical beams.
-
Orthotopic animal models to measure coverage, shed-marker surrogates, lung mechanics, histology, imaging visibility, and partial de-gelation as a safety test.
-
Regulatory exploration as a combination product; this remains a research concept pending data.
Why propose this
Standard therapies focus on destroying tumor cells. This concept asks whether a temporary, well-controlled physical layer at the margins could support that goal by stabilizing the interface and simplifying quality assurance, without adding undue risk. The answer depends on data; this post is an invitation to examine the question with clear criteria.